Introduction to Hydrocarbon Gas Flammability
Today in Part 1 we will introduce important concepts in hydrocarbon gas flammability.
For a fire or deflagration to occur, three things must be present: oxygen (above the minimum oxygen of combustion), an ignition source, and hydrocarbons (in concentrations between the Upper Flammability Limit and Lower Flammability Limit). The fire or deflagration triangle below illustrates that all three are required and equally important. If any one is missing, a fire or deflagration is impossible.

The minimum oxygen of combustion is the level of oxygen required with a given hydrocarbon for a flame to propagate. When you are below this level of oxygen but still have high levels of hydrocarbons and an active ignition source, you will NOT have a deflagration or fire. Typical minimum oxygen of combustion levels for hydrocarbons are between 8% and 12% by volume.
When oxygen and hydrocarbon levels are met, any ignition source is enough to ignite either a fire or deflagration. Ignition sources include: auto-oxidation, auto-ignition, catalytic material, electrical arcing, static charge, friction, compression, coke/carbon, or an external flame source.
Minimum Ignition Energy
The minimum ignition energy is a measure of required energy for a localized ignition source, like a spark, to successfully ignite a fuel-oxidixer mixture. As shown in the figure below, the ignition energy depends on the fuel concentration. For most combustible fuels, the minimum ignition energy is between 0.1 and 0.3 mJ in normal ambient air. However, hydrogen, acetylene and carbon disulfide have one order of magnitude lower minimum ignition energy, Kuchta, 1985 (1).
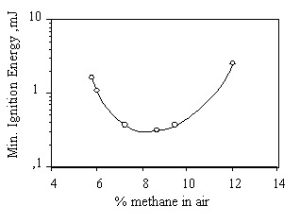
Ignition energy for methane in air at 1 atm. and 25°C.
Autoignition Temperature (AIT)
When a flammable mixture is heated up to a certain temperature, the chemical reaction will start spontaneously. As shown in the figure below, this critical temperature for fuel-oxidizer is called the (minimum) autoignition temperature (AIT). The precise definition is: the autoignition temperature is the lowest temperature of a hot wall adjacent to the fuel-air mixture, which can lead to ignition.
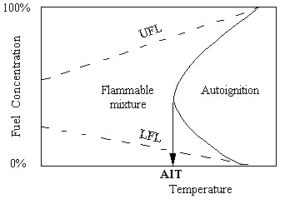
Autoignition Temperature (AIT)
For most pure hydrocarbon derivatives in air, the AIT lies between 540°C (methane) and 210°C (n-decane). For mixtures of hydrocarbons, the AIT lies between the AIT of the pure hydrocarbons, as shown below by Kong and Alfert, 1991 (2).

Autoignition temperature of methane-propane mixture as found in a 1 litre ignition bomb (stoichiometric mixtures). Small vessels show higher than normal AITs.
Freshly delivered asphalt, for example, will have a different autoignition temperature in its vapor space than material that has sat in a tank for 3 days. This is because the chemical composition changes with time as the light hydrocarbons boil (evaporate out).
The Autoignition method we normally use is ASTM E-659. Section X2 of the method says that the larger the sample vessel the lower the AIT. They recommend that the procedure be repeated in 3 or more test volumes such as 250, 500, 1000, and 5000 ml of the same geometry. A plot of autoignition versus logarithm of the vessel volume can be helpful in estimating the AITs at other volumes.
Flammability Limits in Hydrocarbons
Hydrocarbon gas flammability concentrations must be within a certain range for a flame to propagate. The lower limit of this flammability range is defined by the lower flammability limit (LFL). The upper limit of the range is defined by the upper flammability limit (UFL). See diagram below.

As shown in the diagram, when hydrocarbon concentrations are outside the flammability range, a fire or deflagration is NOT possible. The LFL and the UFL are different for each chemical compound. For vapor mixtures, Le Châtelier’s rule is used to calculate composite LFL and UFLs. Flammability limits (LFL, UFL) are typically given at 25 °C (77 °F) since both temperature and pressure effect the determination of LFL and UFLs. See charts below.
Temperature reduces the LFL and increases the UFL widening the Flammable range:

Pressure does not affect the LFL but does increase the UFL and widen the Flammable range:

Flash Points
The flash point of fuel is the minimum temperature at which the fuel gives off sufficient vapor to form a flammable mixture above the liquid within a vessel (vapor has reached LFL). Operating at temperatures lower than the flash point of the liquid fuel will not lead to a flammable mixture being formed unless a mist cloud (e.g. due to splashing) is generated.

Open cup flash points are higher than closed cup flash points and are applicable, for example, to conditions above flammable liquid in open vessels and in spills. The closed cup flash point is the temperature at which the equilibrium concentration of a vapor over a flammable liquid is equal to the lower flammability limit of the vapor.
Vapor Pressure and Raoult’s Law
Each component in a mixture of organic compounds, for example, exerts a certain partial pressure in the vapor space. The more a component is present in the liquid state, the lower the boiling point of this component and the higher the temperature; the more this component will be present in the vapor space.
Raoult’s Law
xi = the mole fraction of a component i in the liquid
yi = the mole fraction of a component i in the vapor space
pi = the partial pressure of component I
P = total pressure in the vapor space
yi = xi pi
Substituting vp(t)/ P
Where vp(t) is the vapor pressure formula (the Antoine Equation) for a given chemical compound as a function of temperature.
yi = xi * vp(t)/ P
Example
The example below we will utilize the Antoine equation for the vapor pressure of dodecane and Raoult’s law to find the temperature at which the vapor in the tank reaches the lower flammability limit.
The chart below shows that as the temperature increases in a tank with pure dodecane, and using Raoult’s law the vapor concentration eventually reaches the lower flammability limit which in this case is 0.62% mole or volume %. This occurred at 135°F.

In the next blog we will discuss dust flammability.
References:
1. Summary of Combustion Properties of Liquid and Gaseous Compounds, KUTCHA, 1985
2. Kong, D., Eckhoff, R.K. and Alfert, F.: Auto-ignition of CH4/air, CH4/C3H8/air and
CH4/CO2/air using a 1 litre ignition bomb. J. Hazard. Materials, Vol. 40 (1995) pp. 69-84
3. Industrial Explosion Prevention and Protection – January 1, 1980, by