|
VAPOUR LIQUID EQUILIBRIA
|
|
Distillation
columns are designed based on the boiling point properties of
the components in the mixtures being separated. Thus the sizes,
particularly the height, of distillation columns are determined
by the vapour liquid equilibrium (VLE) data for the mixtures.
|
|
Vapour-Liquid-Equilibrium (VLE) Curves
|
 Constant
pressure VLE data is obtained from boiling
point diagrams. VLE data of binary mixtures is often presented
as a plot, as shown in the figure on the right. The VLE plot expresses
the bubble-point and the dew-point of a binary mixture at constant
pressure. The curved line is called the equilibrium
line and describes the compositions of the liquid and vapour
in equilibrium at some fixed pressure.
Constant
pressure VLE data is obtained from boiling
point diagrams. VLE data of binary mixtures is often presented
as a plot, as shown in the figure on the right. The VLE plot expresses
the bubble-point and the dew-point of a binary mixture at constant
pressure. The curved line is called the equilibrium
line and describes the compositions of the liquid and vapour
in equilibrium at some fixed pressure.
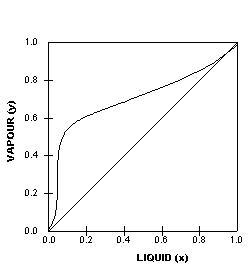
This particular VLE plot shows
a binary mixture that has a uniform vapour-liquid equilibrium
that is relatively easy to separate. The next two VLE plots
below on the other hand, shows non-ideal systems which will
present more difficult separations. We can tell from the shapes
of the curves and this will be explained further later on.
|
|
|
|
The
most intriguing VLE curves are generated by azeotropic systems.
An azeotrope is a liquid mixture which when
vaporised, produces the same composition as the liquid.
The two VLE plots below, show two different azeotropic systems,
one with a minimum boiling point and one with a maximum boiling
point. In both plots, the equilibrium curves cross the diagonal
lines, and this are azeotropic points
where the azeotropes occur. In other words azeotropic systems
give rise to VLE plots where the equilibrium curves crosses the
diagonals.
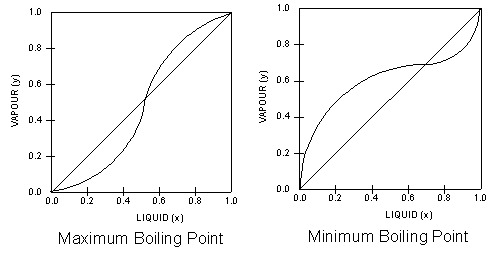
Note
the shapes of the respective equilibrium lines in relation to
the diagonal lines that bisect the VLE plots.
Both
plots are however, obtained from homogenous azeotropic systems.
An azeotrope that contains one liquid phase in contact with
vapour is called a homogenous azeotrope. A homogenous
azeotrope cannot
be separated by conventional distillation. However, vacumn
distillation may be used as the lower pressures can shift
the azeotropic point.Alternatively, an additional substance
may added to shift the azeotropic point to a more ‘favourable’
position.
|

|
When
this additional component appears in appreciable amounts at the
top of the column, the operation is called azeotropic distillation
.
|
 |
When
the additional component appears mostly at the bottom of the column,
the operation is called extractive distillation
|
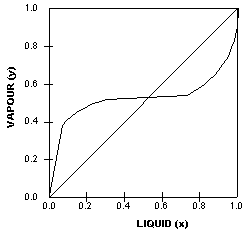 The
VLE curve on the left is also generated by an azeotropic system,
in this case a heterogenous azeotrope.
Heterogenous azeotropes can be identified by the ‘flat’
portion on the equilibrium diagram.
The
VLE curve on the left is also generated by an azeotropic system,
in this case a heterogenous azeotrope.
Heterogenous azeotropes can be identified by the ‘flat’
portion on the equilibrium diagram.
They
may be separated in 2 distillation columns since these substances
usually form two liquid phases with widely differing compositions.
The phases may be separated using settling tanks under appropriate
conditions.
|
|
Next,
we will look at how VLE plots/data are used to design distillation
columns.
|

